News & Media
template_vision_en

Genetic disorders and gene therapy
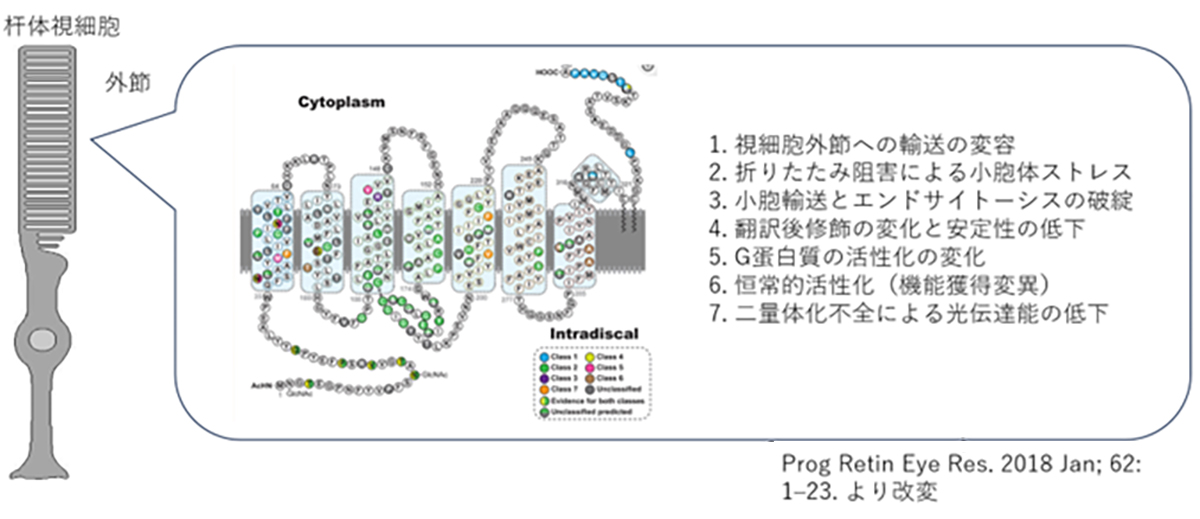
VC Gene Therapy, Inc. was established in August 2020 as a subsidiary of Vision Care, Inc. (certified by RIKEN Ventures). We are responsible for the development of gene therapies, which is one of Vision Care Group’s branches. Currently, we are progressing in the development of gene therapies for autosomal dominant inherited retinal degenerative diseases using genome editing, aiming for clinical trials in two years. Retinitis pigmentosa is one of the inherited retinal dystrophies (IRD) and is a hereditary, progressive disease that affects the vision cells and the retinal pigment epithelium (RPE) in the outer layer of the retina, leading to visual impairments. In Japan, it is the second most common visual impairment after glaucoma and is one of the designated intractable diseases recognized by the Ministry of Health, Labour and Welfare. While nearly 200 causative genes have been reported and cases have been accumulating, there are still causative genes that have yet to be identified, and research is being conducted to establish early treatments.
Gene therapy for retinitis pigmentosa
At VCGT Inc., we are primarily developing treatments targeting RHO (rhodopsin) gene mutations, which are categorized as the dominant inheritance type and has the highest number of patients among those with retinitis pigmentosa. Furthermore, we are also focusing on developing therapies that can be applied to other causative genes, with the RHO gene at the center of this work. Up to this point, our predecessor, the Retinal Regeneration Project at RIKEN, and the Kobe City Eye Center Hospital have made significant strides in understanding retinitis pigmentosa. They have focused on unraveling the causative gene, carrying out gene diagnosis and genetic counseling accessible in outpatient clinics, and establishing a database to manage the clinical information of patients.